Staphylococcus
Staphylococcus
Staphylococcus is a genus of gram positive, non-motile bacteria which are round in shape and form cluster like grapes. This genus includes at least 40 species of which S. aureus is the cause of food poisoning. The illness is the result of the consumption of the Staphylococcus enterotoxin which is produced by toxigenic strains of S. aureus. Staphylococcus can also cause illness due to direct infection like boils, impetigo, and cellulitis. Due to the high prevalence of this bacteria all kinds of food have been associated with the outbreaks of this form of food poisoning but dairy products, salads, eggs meat are common. The bacteria have low resistance to heat, however SET has almost total resistance to dehydration, proteolytic enzymes, and heat treatment. SET detection is costly and tedious and traditional methods of detection level of detection is not sensitive enough.
Occurrence of Staphylococcus
S. aureus occurs commonly in the environment and can be transmitted through the air in the form of droplets as a person sneeze. 30% of people carry these bacteria in their noses back of the throat and on their skin. In animals, these bacteria can cause mastitis and they can infect milk through this route. Of late some strains of this bacteria has developed resistance to the antibiotic Methicillin known as Methicillin resistant Staphylococcus aureus (MRSA).
Convetional Method - Biolife Italiana
Arasains Indonesia distributes Biolife culture media for the detection of Staphylococcus. The gold standard for the enumeration of coagulase positive staphylococci is culturing with Baird Parker agar. S aureus is the only coagulase and thermonuclase producing staphylococcus which is also reduces tellurite. This reaction is used in the selective Baird Parker agar. Staphylococcus aureus produces lipase in the BPA agar to form grey to black colonies with characteristic zones of clearing in BPA with egg yolk. Another agar is rabbit fibrinogen agar.
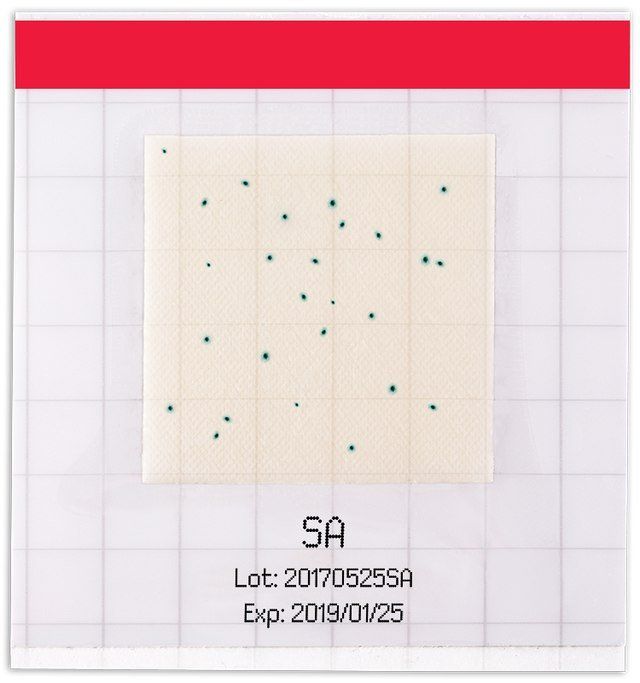
Film Method – MC-Media Pad® Staphylococcus aureus
Merck Indonesia supplies the MC-Media Pad® that designed for convenient and rapid routine testing of microbial contaminations in your food and beverage products. MC-Media Pad® S. aureus is a convenient culture media for enumeration of Staphylococcus aureus.
Benefits:
- Save space in your fridge and incubator
- Go green and gain: reduce your environmental impact
- Improve your inventory management with shelf life of up to 15 months.
- Comply with regulations.
- Simply your workflow on product testing and environmental testing.
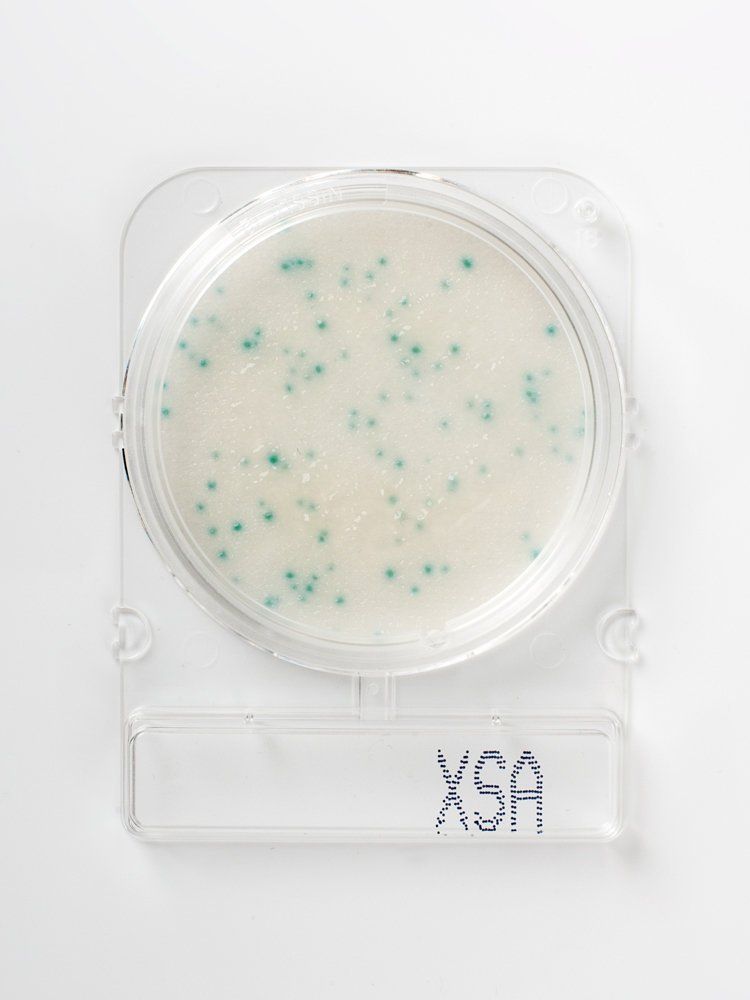
Film Method – Compact Dry X-SA
Usage of Compact Dry X-SA is a simple and safe test procedure for determination and quantification of Staphylococcus aureus in foods, cosmetics or raw materials – as well as pharmaceutical raw materials. The ready-to-use plates consist of a special 50 mm diameter petri dish containing a detection specific nutrient pad. Compact Dry X-SA detects S. aureus after only 24 h incubation time at 37 °C. The bright blue colonies are easy to identify on the nutrient pad and therefore easy to evaluate.
Benefits:
- Ready-to-use
- No change in handling
- No additional tools & equipment
- Approved by AOAC, NordVal and MicroVal
- Reliable results, easy to read and count
- Suitable for food samples, liquids & surface swabs
- Easy to store at RT
ELISA – TRANSIA™PLATE Staphylococcus enterotoxin
Merck Biocontrol Indonesia supplies a TRANSIA™PLATE microtiter plate detection method for
Staphylococcus enterotoxin. Transia plate test kit is a sandwich enzyme immunoassay for the detection of
Staphylococcus enterotoxin A, B, C1, C2, C3, D and E in food as well as in bacterial culture. This unique combination of monoclonal and polyclonal antibodies makes this method highly specific and sensitive. Laboratory automation is possible with the use of Gemini auto washer incubator and reader.
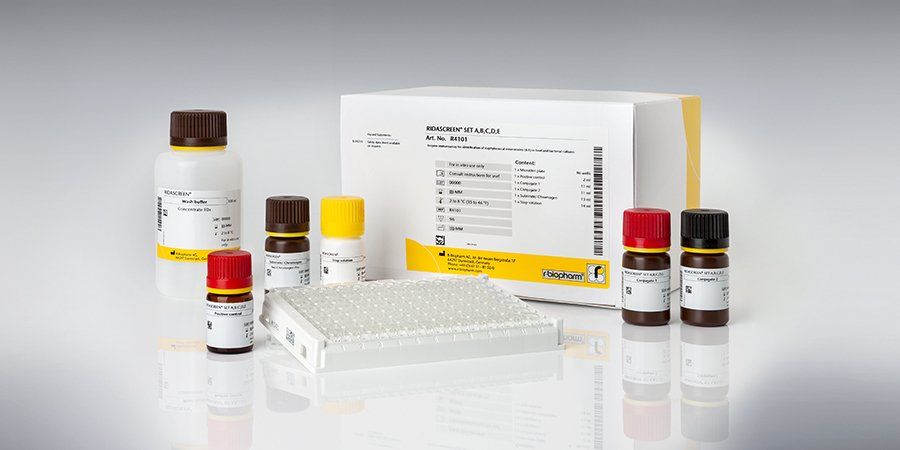
ELISA – RIDASCREEN® SET A, B, C, D, E
RIDASCREEN®
SET A,B,C,D,E is a sandwich enzyme immunoassay for the identification of Staphylococcus enterotoxins A, B, C, D and E in fluid and solid foods as well as in bacterial cultures. Based on its sensitivity the RIDASCREEN® SET A,B,C,D,E test is consequently clear superior to the immunodiffusion procedure which has a detection limit of 0.1 mg / ml.